
If you have any questions, please contact: Centric@soton.ac.uk
This page will help you decide which data you would like to analyse.
Overview of HORIZONS data
Data sources:
- Self-completion questionnaire data completed by participants at six time points
- Case Report Form (CRF) data completed by staff at the HORIZONS recruiting hospitals at five time points
Data time points:
| Timepoint | Questionnaire data | CRF data |
| Baseline* ** | X | X |
| 3 months post consent** | X | |
| 6 months post consent | X | |
| 12 months post consent | X | X |
| 18 months post consent | X | |
| 24 months post consent | X | X |
| 36 months post consent | X | X |
*Baseline questionnaire data, where possible, was completed before the start of primary cancer treatment.
**The first six months of HORIZONS was the pilot phase of the study. Feedback from participants and partners gathered in the pilot phase informed updates to the baseline and 3 month questionnaires.
HORIZONS questionnaire content
Summary:
- Participants’ background data (sociodemographic and economic data)
- General health and wellbeing of participants
- Symptoms and how participants are feeling
- How participants cope and manage their health
- Participants’ interests
- The help and support available to participants
- Participants’ diet and lifestyle
- Participants’ comments and miscellaneous questions
Further details:
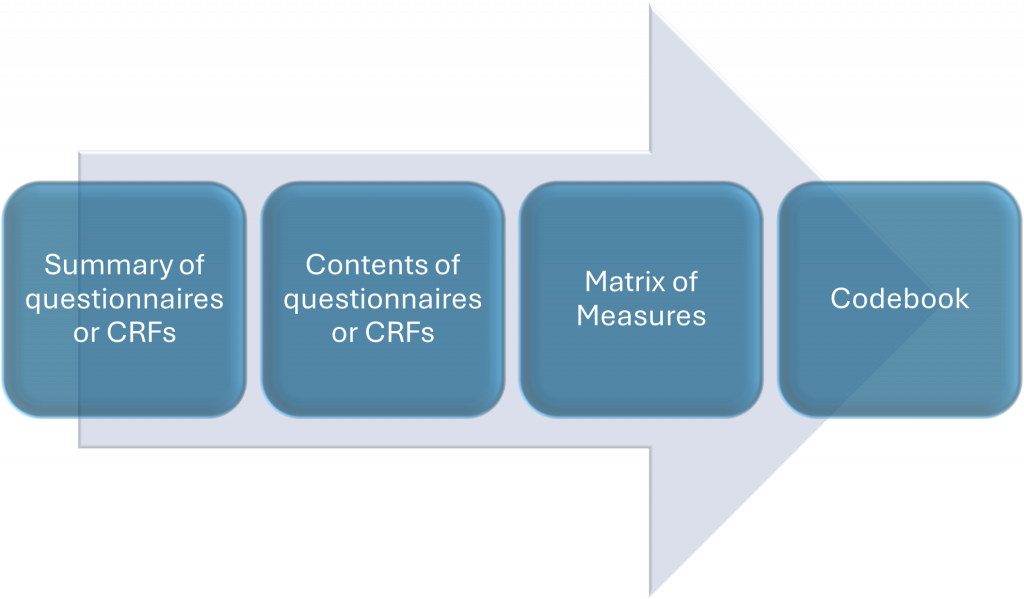
You can find further details of the individual questions asked and the validated measures used by looking at:
The ‘Contents’ documents for each questionnaire
(Baseline (PILOT), 3 Month (PILOT), Baseline, 3 Month, 12 Month, 18 Month, 24 Month, 36 Month)
The ‘Matrix of Measures’ which shows all questions and measures and the time points at which they were included
Data Collection Tools
Study Questionnaires

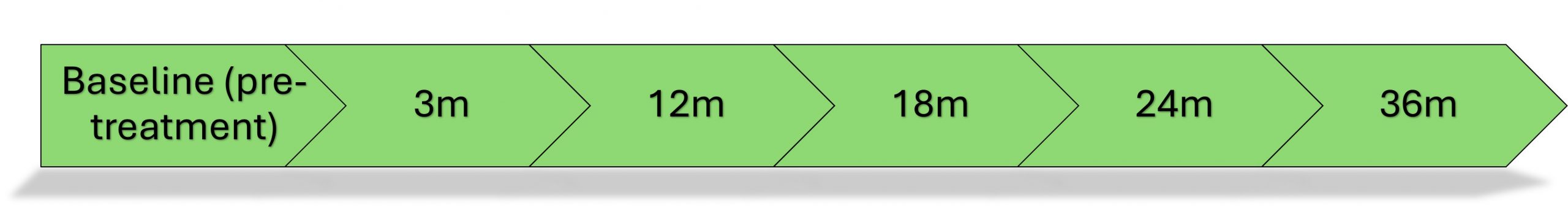
Participants completed questionnaires at multiple time points: before treatment, then at 3, 12, 18, 24 and 36 months after consent. Details of questions asked are available in the Matrix of Measures.
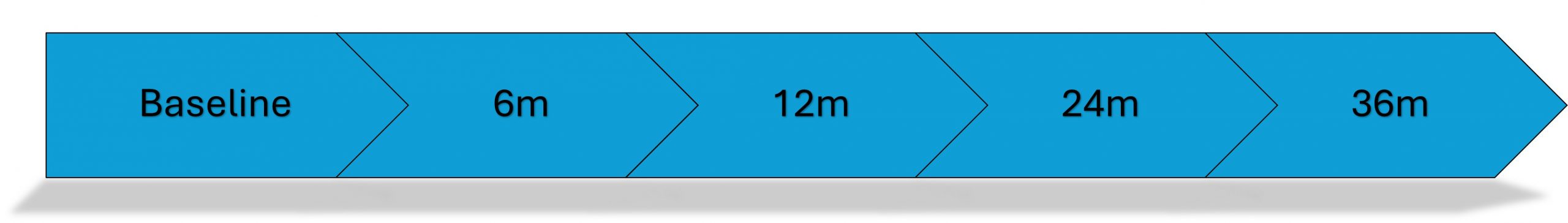
Case Report Forms (CRFs)
Participants complete questionnaires at multiple time points: before treatment, then at 3, 12, 18, 24 and 36 months after consent.
| Cancer Type | Baseline | 6mo | 12mo | 24mo | 36mo |
|---|---|---|---|---|---|
| Breast cancer | view | view | view | view | view |
| NHL | view | view | view | view | view |
| Cervical cancer | view | view | view | view | view |
| Endometrial cancer | view | view | view | view | view |
| Ovarian cancer | view | view | view | view | view |
| Vulval cancer | view | view | view | view | view |
If you have further questions about this process please contact us via Centric@soton.ac.uk.